1. Free Radical Chemistry: A New Approach to Synthesis
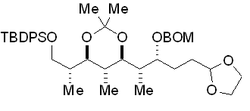
One of the long-term objectives of our research program is to contribute at strategic, tactical and theoretical levels with approaches leading to the creation of new stereogenic centers, notably on acyclic molecules, which as a class are among the most difficult chemical entities to transform stereoselectively because of their flexibility. Of particular interest to us is the use of free-radical intermediates that were completely overlooked until a decade ago, in such approaches.
2. Chemistry of Sugars: Synthesis of N-Glycosides
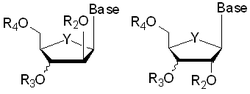
The goal of this research program is the development of synthetic strategies that permit facile, stereocontrolled access to analogues of purine and pyrimidine nucleosides. Inspired by the structure of N-glycosides this project could lead to the discovery of new molecular prototypes. N-glycosides (natural or synthetic) and their acyclic derivatives are biologically significant for a number of reasons. These compounds are found to exhibit a variety of therapeutically-promising properties which include antitumor, antibacterial and antiviral activities.
3. Chemistry of Carbohydrates: Sialyl Lewis X Mimetics
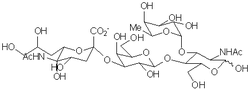
The present research program stems from our interest in what could appear to be unrelated scientific issues: the discovery of new synthetic methodologies that allow for diastereoselective modifications of acyclic free radical intermediates, and the development of sialyl Lewis X (sLeX) mimetics as antagonists of E- and P-selectins. The latter originates from our interest in altering the biological effects mediated by the interaction between posttranslationally modified proteins (e.g., glycoproteins) and their receptors.
