Publications
127. ''Synthesis of 4′-Thionucleoside Analogues Bearing a C2′ Stereogenic All-Carbon Quaternary Center'' Eymard, C.; Manchoju, A.; Almazloum, A.; Dostie, S.; Prévost, M.; Nemer, M.; Guindon, Y. Synthesis of 4′-Thionucleoside Analogues Bearing a C2′ Stereogenic All-Carbon Quaternary Center. Molecules 2024, 29, 1647. https://doi.org/10.3390/molecules29071647
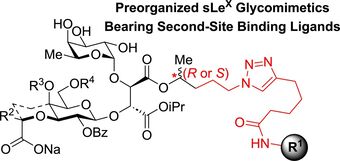
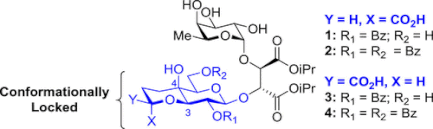
126. "Sialyl LewisX glycomimetics bearing an extended anionic chain targeting E- and P- selectin binding sites." Belouin, A., Simard, R. D., Joyal, M., Maharsy, W., Lau, A., Prévost, M., Nemer, M., and Guindon, Y. Bioorg. Med. Chem. 2024, 98, 117553.
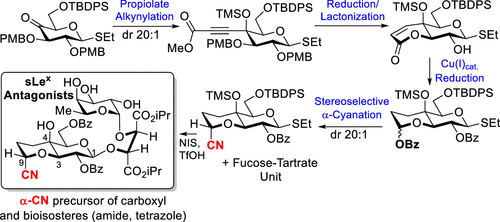
125. "Synthesis of Sialyl Lewis X Miimetics with E-and P- Selectin Binding Properties and Immunosuppressive Activity". Simard, R., Joyal, M., Beaugrand, T., Gauthier,J., Hardine, M., Desriac, A., Buffet, C.H., Prévost, M. Nemer, M., Guindon, Y., Journal of Organic Chemistry, 2023, Https://doi.org./10.1021/acs.joc.3c00956
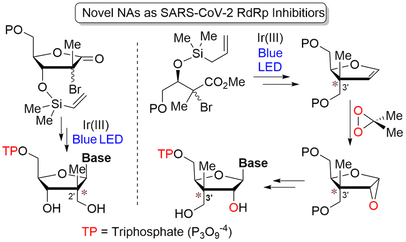
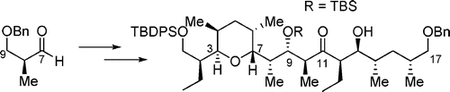
124. "Nucleotide Analogues Bearing a C2' or C3'-Stereogenic All-carbon Quaternary Center as SARS-CoV-2". Manchoju A.; Zelli R.; Wang G.; Eymard C.; Oo Adrian.; Nemer M.; Prévost M.; Baek K. and Guindon Y. Molecules 2022, 27, 594.
123. "Diastereoselective and Regioselective Synthesis of Adenosine Thionucleoside Analogues Using an Acyclic Approach". Cardinal-David B., Labbé, M.O., Prévost, M., Dostie S., and Guindon, Y. Can. J. of Chem., 2020,98(9), 466-470 (Commemorative issue in celebration of the 100th anniversary of the Université de Montréal's Department of Chemistry).
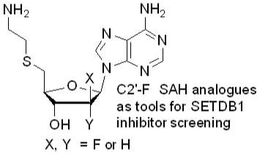
122. "Identification of a C2'-Fluorinated SAH Analogue". Labbé, M.O., Li, F., Chau, I., Xiong, Z-J.,Santhakumar, V., Dostie, S., Guindon, Y., Can. J. of Chem., 2020, 98(6), 318-321(Commemorative issue in Honor of Dr. James Wuest).
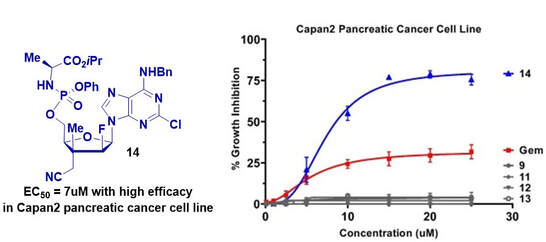
121. "Identification of a C3’-nitrile nucleoside analogue inhibitor of pancreatic cancer cell line growth." Labbé, M.-O.; Collins, L.; Lefebvre, C.-A.; Maharsy, W.; Beauregard, J.; Dostie, S.; Prévost, M.; Nemer, M.; Guindon, Y., Bioorganic & Medicinal Chemistry Letters, 2020, 30,(6), 126983.
120. "Diastereoselective Synthesis of Arabino- and Ribo-like Nucleoside Analogues Bearing a Stereogenic C3′ All-Carbon Quaternary Center." Lussier, T.; Manchoju, A.; Wang, G.; Dostie, S.; Foster, S.; Mochirian, P.; Prévost, M.; Guindon, Y., J. Org. Chem., 2019, 84, 16055-16067
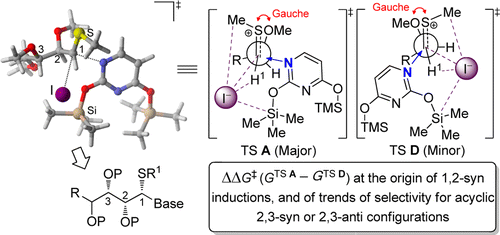
119. "Photoredox-Catalyzed Stereoselective Radical Reactions to Synthesize Nucleoside Analogues with a C2'-stereogenic All-carbon Quaternary Center", F. Becerril-Jiménez, T. Lussier, L. Leblanc, C. Eymard, S. Dostie, M. Prévost, Y.Guindon, J. Org Chem., 2019, 84, 14795-14804.
118. “Synthesis of Sialyl LewisX Glycomimietics Bearing a Bicyclic 3-O,4-C Fused Galactopyranoside Scaffold”, R.D. Simard, M. Joyal, L. Gillard, G. Di Censo, W. Maharsy, J. Beauregard, P. Colarusso, K. D. Patel, M. Prévost, M. Nemer, Y. Guindon. J. Org. Chem., 2019, 84, 7372-7387.
117. “Synthesis of Nucleoside Analogues using Acyclic Diastereoselective reactions”, T. Lussier, M.-E. Waltz, G. Freure, P. Mochirian, S. Dostie, M. Prévost and Y. Guindon, Arkivoc, 2019, part iv, 113-142 (Commemorative Issue in Honor of Dr. Stephen Hanessian on the occasion of his 84th anniversary).
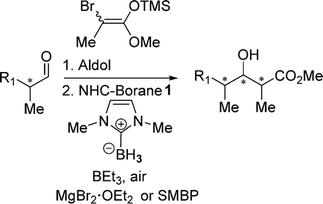
116. ''Diastereoselective Radical Hydrogen Transfer Reactions using N-Heterocyclic Carbene Boranes.'' Tambutet, G.; Guindon, Y., J. Org. Chem. 2016, 81(22), 11427-11431.
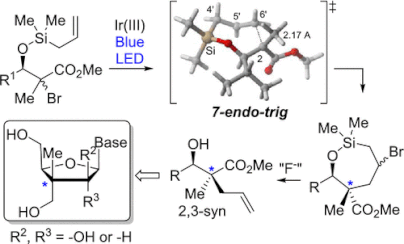
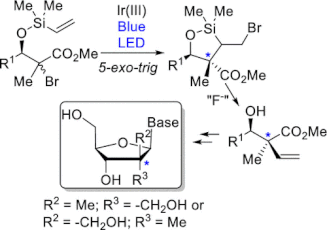
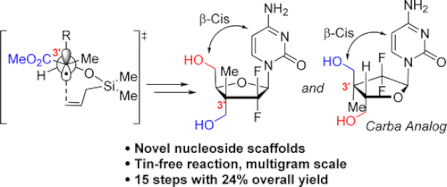
115. ''Diastereoselective Synthesis of C2′-Fluorinated Nucleoside Analogues Using an Acyclic Approach.'' Dostie, S.; Prévost, M.; Mochirian, P.; Tanveer, K.; Andrella, N.; Rostami, A.; Tambutet, G.; Guindon, Y., J. Org. Chem. 2016, 81(22) 10769-10790.
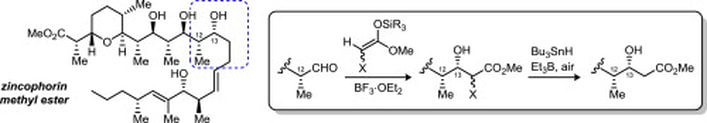
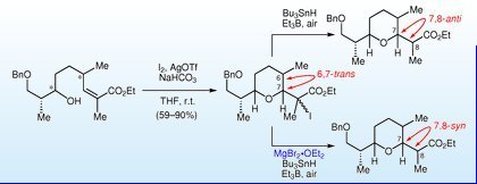
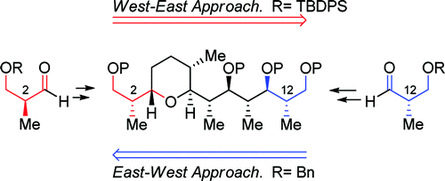
114.''Total synthesis of zincophorin methyl ester. Stereocontrol of 1,2-induction using sterically hindered enoxysilanes.'' Godin, F.; Mochirian, P.; St-Pierre, G.; Guindon, Y., Tetrahedron 2015, 71 (4), 709-726.
113. ''Dual-Face Nucleoside Scaffold Featuring a Stereogenic All-Carbon Quaternary Center. Intramolecular Silicon Tethered Group-Transfer Reaction.'' Tambutet, G.; Becerril-Jiménez, F.; Dostie, S.; Simard, R.; Prévost, M.; Mochirian, P.; Guindon, Y., Org. Lett 2014, 16, 5698-5701.
112. ''Investigation of Diastereoselective Acyclic α-Alkoxydithioacetal Substitutions Involving Thiacarbenium Intermediates.'' Prévost, M.; Dostie, S.; Waltz, M.-È.; Guindon, Y., J. Org. Chem. 2014, J. Org. Chem., 79 (21), 10540-10525.
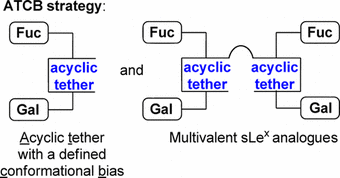
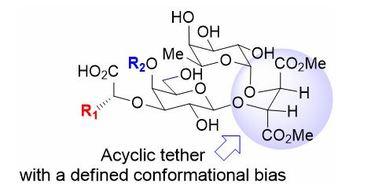
111. "Acyclic Tethers Mimicking Subunits of Polysaccharide Ligands : Selectin Antagonists". M. Calosso, G. Tambutet, D. Charpentier, G. St-Pierre, M. Vaillancourt, M. Bencheqroun, J.F. Gratton, M. Prévost, Y. Guindon, ACS Medicinal Chemistry Letters., 2014, 5, 1054-1059
110. "Stereocontrolled Synthesis of Propionate Motifs from L-lactic and L-alanine Aldehydes. A DFT Study of the Hydrogen Transfer Under Endocyclic Control". F. Godin, M. Duplessis, C. Buonomano, T. Trinh, K. Houde, D. Chapdelaine, J. Rodrigue, A. Boutros and Y. Guindon,. Org. Chem. Front, 2014, 1(8) 974-982.
109. "A Study of Exocyclic Radical Reductions of Polysubstituted Tetrahydropyrans" F. Godin, M. Prévost, F. Viens, P. Mochirian, J.-F. Brazeau, S. I. Gorelsky et Y. Guindon J. Org. Chem 2013, 78(12), 6075-6103.
108. "A Study of the Endocyclic versus Exocyclic C–O Bond Cleavage Pathways of alpha- and beta-Methyl Furanosides" O. St-Jean, M. Prévost, Y. Guindon J. Org. Chem. 2013, 78(7), 2935-2946.
107. "Diastereoselective hydrogen transfer reactions: An experimental and DFT study" F. Godin, M. Prévost, S. I. Gorelsky, P. Mochirian, M. Nguyen, F. Viens and Y. Guindon. '' Chem. Eur. J. 2013, 19(28), 9308-9318.
106. “A New Approach to Explore the Binding Space of Polysaccharide-based Ligand: Selectin Antagonist" M. Calosso, D. Charpentier, M. Vaillancourt, M. Bencheqroun, G. St-Pierre, B.C. Wilkes, Y. Guindon. ACS Medicinal Chemistry Letters 2012, 3, 1045.
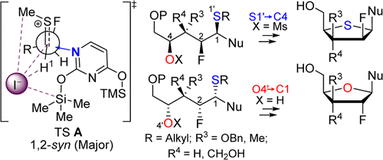
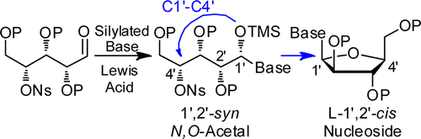
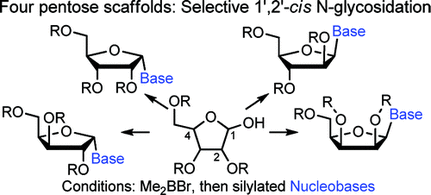
105. “A Stereoselective Approach to b-L-Arabino Nucleoside Analogues: Synthesis and Cyclization of Acyclic 1’,2’-syn N,O-Acetals”, S. Dostie, M. Prévost, Y. Guindon J. Org. Chem 2012, 77, 7176. (Featured Article)
104. “Stereodivergent Synthesis of the C1-C9 Tetrahydropyran Subunit of Zincophorin and Isomers Thereof”, F. Godin, I. Katsoulis, E. Fiola-Masson, S. Dhambri, P. Mochirian, Y. Guindon; Synthesis 2012, 44, 474.
103. "Selected Diastereoselective Reaction- Free Radical Additions and cyclization", Y. Guindon, F. Godin, P. Mochirian, M. Prévost. In Comprehensive Chirality, H. Yamamoto, E. Carreira; Eds. Elesevier Oxford, UK, Guindon, 2012.
102. “A Bidirectional Approach to the Synthesis of Polypropionates Synthesis of C1 C13 Fragment of Zincophorin and Related Isomers”, P. Mochirian, F. Godin, I. Katsoulis, I. Fontaine, J.F. Brazeau, Y. Guindon; J. Org. Chem. 2011, 76, 7654. (Featured Article).
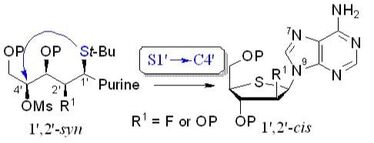
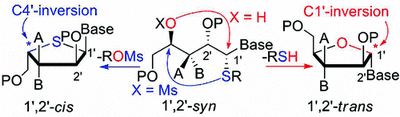
101. “Synthesis of 1′,2′-cis-Nucleoside Analogues: Evidence of Stereoelectronic Control for SN2 Reactions at the Anomeric Center of Furanosides”, M. Prévost, O. St-Jean, Y. Guindon; J. Am. Chem. Soc., 2010, 132, 12433.
100. “Stereocontrolled Synthesis of C1-C17 Fragment of Narasin via a Free Radical-Based Approach”, J.-F. Brazeau, A.-A. Guilbault, J. Kochuparampil, P. Mochirian, Y. Guindon; Org. Lett. 2010, 12, 36.
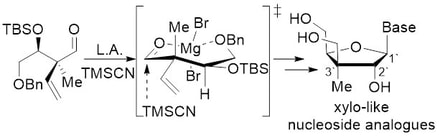
99. “A Stereoselective Approach to Nucleosides and 4’-thioanalogues from Acyclic Precursors”, D. Chapdelaine, B. Cardinal-David, M. Prévost, M. Gagnon, I. Thumin, Y. Guindon; J. Am. Chem. Soc. 2009, 131, 17242.
98. “Stereoselective Quaternary Center Construction via Atom-Transfer Radical Cyclization using Silicon Tethers on Acyclic Precursors”, M. Duplessis, B. Cardinal-David, M.-E. Waltz, Y. Guindon; Org. Lett. 2009, 11, 3148.
97. “Raising the Ceiling of Diastereoselectivity in Hydrogen Transfer on Acyclic Radicals”, I. Denissova, L. Maretti, B.C. Wilkes, J.C. Scaiano, Y. Guindon; J. Org. Chem. 2009, 74, 2438.
96. “Stereopentads Derived from a Sequence of Mukaiyama Aldolization and Free Radical Reduction on a-Methyl-b-Alkoxy Aldehydes: A General Strategy for Efficient Polypropionate Synthesis”, J.-F. Brazeau, P. Mochirian, M. Prévost, Y. Guindon; J.Org. Chem., 2009, 74, 64.
Publications
Books Chapters
09. “Selected Diastereoselective Reaction – Free Radical Additions and cyclizations”, Y. Guindon, F. Godin, P. Mochirian, M. Prévost. (Book Chapter Accepted). In Comprehensive Chirality, H. Yamamoto, E. Carreira; Eds. Elsevier: Oxford, UK, Guindon (2012).
08. "Lewis Acid-Mediated Diastereoselective Radical Reactions". B. Guérin, W.W. Ogilvie, Y. Guindon; Radicals in Organic Synthesis, Volume 1, P. Renaud, M.P. Sibi (Eds), Wiley-VCH Verlag GmbH: Weinheim, 2001, pp.441-460.
07. "Encyclopedia of Reagents for Organic Synthesis":
1. "Bromo (t-Butyl) (Methoxy) Phenylsilane". Y. Guindon and C. Yoakim;
2. "β-Bromocatecholborane". P. Anderson and Y. Guindon;
3. "Bromodimethylborane". Y. Guindon and P. Anderson;
4. "t-Butoxychlorodiphenylsilane". Y. Guindon and C. Yoakim;
Ed. Leo Paquette, John Wiley & Sons, Sussex, England, 1995.
06. "Therapeutic Potential of 5-Lipoxygenase Inhibitors: The Discovery and Development of MK-886, A Novel-Mechanism Leukotriene Inhibitor". J. W. Gillard, R. Dixon, D. Ethier, J. Evans, A. W. Ford-Hutchinson, R. Fortin, Y. Girard, Y. Guindon, P. Hamel, T. Jones, C. Léveillé, A. Lord, D. Miller, H. E. Morton, C. Rouzer and C. Yoakim; Side-Effects of Anti-inflammatory Drugs 3. Inflammation and Drug Therapy Series; K. D. Rainsford and G. P. Velo (Eds), Kluwer Academic Publishers: Boston, 1992; pp. 275-286.
05. "Chemistry of the Leukotrienes and Other Lipoxygenase Products". C. K. Lau, J. Adams, Y. Guindon and J. Rokach; Leukotrienes and Lipoxygenases, p. 1-130, Ed. J. Rokach, Elsevier, New York, 1989.
04. "Tetrahydrocarbazol-1-Acetic Acids: New Class of Thromboxane Receptor Antagonists". Y. Girard, C. Yoakim-Rancourt, P. Hamel, J. W. Gillard, Y. Guindon, G. Letts, J. Evans, C. Léveillé, D. Ethier, A. Lord, T. Jones, P. Masson, A. W. Ford-Hutchinson and J. Rokach; Prostaglandins in Clinical Research: Cardiovascular System, p. 585-589, Alan R. Liss, Inc., New York, 1989.
03. "The Discovery and Optimization of New Classes of Thromboxane Antagonists and Leukotriene Biosynthesis Inhibitors". J. G. Gillard, Y. Girard, H. E. Morton, C. Yoakim, R. Fortin, Y. Guindon, T. R. Jones, A. Lord, D. Ethier, et al.; New Trends in Lipid Mediators Research, Vol. 3. Leukotrienes and Prostanoids in Health and Disease, p. 46-49, S. Karger, Basel, Switzerland, 1989.
02. "Synthesis of the Leukotrienes". J. Rokach, Y. Guindon, R. N. Young, J. Adams and J. G. Atkinson; The Total Synthesis of Natural Products, Vol. 7, p. 141-274, Ed. John W. ApSimon, Wiley Interscience, New York, 1988.
01. "Leukotrienes: Biological and Chemical Importance". New Methods in Drug Research, Vol. 2, p. 195-213. A. Makriyannis, J.R. Prous (Eds), Science Publ., Spain, 1988.
08. "Lewis Acid-Mediated Diastereoselective Radical Reactions". B. Guérin, W.W. Ogilvie, Y. Guindon; Radicals in Organic Synthesis, Volume 1, P. Renaud, M.P. Sibi (Eds), Wiley-VCH Verlag GmbH: Weinheim, 2001, pp.441-460.
07. "Encyclopedia of Reagents for Organic Synthesis":
1. "Bromo (t-Butyl) (Methoxy) Phenylsilane". Y. Guindon and C. Yoakim;
2. "β-Bromocatecholborane". P. Anderson and Y. Guindon;
3. "Bromodimethylborane". Y. Guindon and P. Anderson;
4. "t-Butoxychlorodiphenylsilane". Y. Guindon and C. Yoakim;
Ed. Leo Paquette, John Wiley & Sons, Sussex, England, 1995.
06. "Therapeutic Potential of 5-Lipoxygenase Inhibitors: The Discovery and Development of MK-886, A Novel-Mechanism Leukotriene Inhibitor". J. W. Gillard, R. Dixon, D. Ethier, J. Evans, A. W. Ford-Hutchinson, R. Fortin, Y. Girard, Y. Guindon, P. Hamel, T. Jones, C. Léveillé, A. Lord, D. Miller, H. E. Morton, C. Rouzer and C. Yoakim; Side-Effects of Anti-inflammatory Drugs 3. Inflammation and Drug Therapy Series; K. D. Rainsford and G. P. Velo (Eds), Kluwer Academic Publishers: Boston, 1992; pp. 275-286.
05. "Chemistry of the Leukotrienes and Other Lipoxygenase Products". C. K. Lau, J. Adams, Y. Guindon and J. Rokach; Leukotrienes and Lipoxygenases, p. 1-130, Ed. J. Rokach, Elsevier, New York, 1989.
04. "Tetrahydrocarbazol-1-Acetic Acids: New Class of Thromboxane Receptor Antagonists". Y. Girard, C. Yoakim-Rancourt, P. Hamel, J. W. Gillard, Y. Guindon, G. Letts, J. Evans, C. Léveillé, D. Ethier, A. Lord, T. Jones, P. Masson, A. W. Ford-Hutchinson and J. Rokach; Prostaglandins in Clinical Research: Cardiovascular System, p. 585-589, Alan R. Liss, Inc., New York, 1989.
03. "The Discovery and Optimization of New Classes of Thromboxane Antagonists and Leukotriene Biosynthesis Inhibitors". J. G. Gillard, Y. Girard, H. E. Morton, C. Yoakim, R. Fortin, Y. Guindon, T. R. Jones, A. Lord, D. Ethier, et al.; New Trends in Lipid Mediators Research, Vol. 3. Leukotrienes and Prostanoids in Health and Disease, p. 46-49, S. Karger, Basel, Switzerland, 1989.
02. "Synthesis of the Leukotrienes". J. Rokach, Y. Guindon, R. N. Young, J. Adams and J. G. Atkinson; The Total Synthesis of Natural Products, Vol. 7, p. 141-274, Ed. John W. ApSimon, Wiley Interscience, New York, 1988.
01. "Leukotrienes: Biological and Chemical Importance". New Methods in Drug Research, Vol. 2, p. 195-213. A. Makriyannis, J.R. Prous (Eds), Science Publ., Spain, 1988.